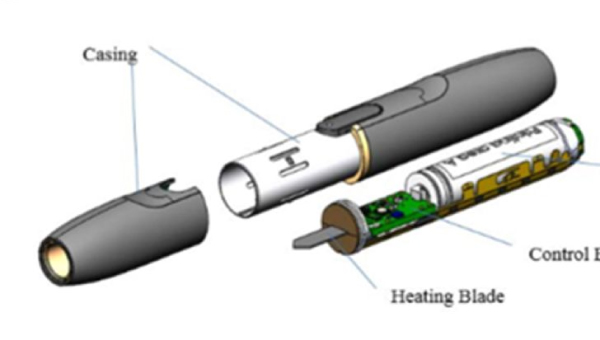
IQOS toxicological constituents mean US FDA should not approve IQOS as a modified risk tobacco product
Public comment by Peyton Jacob III, PhD; Lauren Kass Lempert, JD, MPH; Stanton A. Glantz, PhD; Bonnie Halpern-Felsher, PhD; Pamela Ling, MD, MPH to the US FDA: Iqos should not be approved as a modified risk tobacco product; Philip Morris has submitted an application for IQOS as a modified risk tobacco product but has failed to include complete and new studies showing substantial toxicological exposures from multiple chemicals known to be carcinogenic. Consequently, Glantz and colleagues argue the FDA must not grant a modified risk approval to IQOS 2.4 or IQOS 3.0. They state,
“IQOS 3 and IQOS 2.4 in fact expose users to significantly increased levels of several dangerous harmful and potentially harmful constituents (HPHCs) and other toxins including carcinogens.”
https://tinyurl.com/22tat92p
—
Stephen Hamann