US FDA Authorizes More Heated Tobacco Products, Rejects More Vapes
Norcia A, Filter magazine. FDA authorizes more heated tobacco products, rejects more vapes. 27 Jan 2023,On January 26, the Food and Drug Administration (FDA) authorized three heated tobacco products manufactured by Philip Morris International. The agency issued marketing granted orders (MGOs) for three tobacco-flavored heat sticks—Marlboro Sienna, Marlboro Bronze, and Marlboro Amber, all of which can be used with PMI’s IQOS device.
https://filtermag.org/fda-heated-tobacco-products/
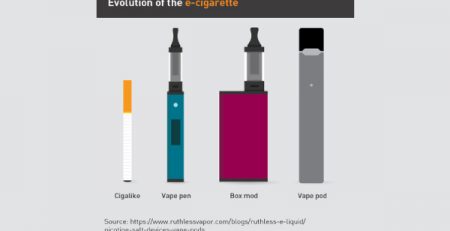
The Vaping Magazine, Filter, notes that electronic cigarettes are not getting marketing approval, while heated tobacco products for Iqos are getting approval. The article notes, “Taiwan just banned the use of vapes” and that “no flavored vape product has gotten approval in the US.” It concludes that the approval process “favors well-financed manufacturers, mainly with ties to the tobacco industry.”
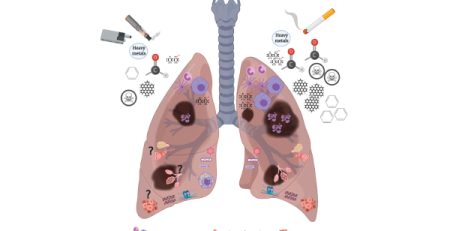
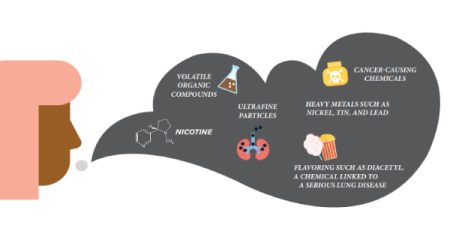
Comment: The vaping industry is alarmed that they can’t get marketing approval, while heated tobacco products (HTPs) seem to get approval. Is it about the money to influence regulation or that vaping has an established record of adverse health effects on users, especially youth who get nicotine addicted and poisoned from flavored vapes?
Stephen Hamann